44 fda approved health claims on food labels
Questions and Answers on Health Claims in Food Labeling | FDA The Nutrition Labeling and Education Act of 1990 (NLEA) directed FDA to issue regulations providing for the use of health claims. All health claims must undergo review by the FDA through a petition process. Health claims: must contain the elements of a substance and a disease or health-related condition; Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Abstract The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA).

Fda approved health claims on food labels
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and Introduction to Food Product Claims — FDA Reader There are two types of health claims that appear on food labels and marketing. They are: Authorized Health Claims Qualified Health Claims Requirements for a Health Claim Health claims cannot be made about the diagnosis, cure, mitigation or treatment of diseases (this is a drug claim) They must be complete, truthful and not misleading. Qualified Health Claims | FDA - U.S. Food and Drug Administration The process does not involve rulemaking. For more information, visit Questions and Answers: Qualified Health Claims in Food Labeling or explore the linked pages below. Qualified Health Claims:...
Fda approved health claims on food labels. The FDA Wants to Update the Definition for "Healthy" Claims on Food Labels Nuts and seeds, fatty fish such as salmon, certain oils and water are currently categories of food that would not be able to be labeled as healthy with this proposed definition. But the FDA notes ... Label Claims for Conventional Foods and Dietary Supplements There are three ways in which FDA exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990... FDA Label Search - Food and Drug Administration Please be aware of the following when using information from this Web site: The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by FDA. Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA). This means there is a consensus in the publically available scientific information on the matter.
FDA Proposes to Update Definition for "Healthy" Claim on Food Labels The "healthy" claim can act as a quick signal on food package labels to help empower consumers, including those with lower nutrition knowledge, with information to identify foods that will help... A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... FDA proposes updates to 'healthy' claim on food packages | CNN About 5% of foods are labeled as being healthy, which is a regulated claim. Foods that make the claim have limits on individual nutrients like fat, saturated fat, cholesterol and sodium, and they ... Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...
Qualified Health Claims | FDA - U.S. Food and Drug Administration The process does not involve rulemaking. For more information, visit Questions and Answers: Qualified Health Claims in Food Labeling or explore the linked pages below. Qualified Health Claims:...
Qualified Health Claims | FDA - U.S. Food and Drug Administration The process does not involve rulemaking. For more information, visit Questions and Answers: Qualified Health Claims in Food Labeling or explore the linked pages below. Qualified Health Claims:...
Introduction to Food Product Claims — FDA Reader There are two types of health claims that appear on food labels and marketing. They are: Authorized Health Claims Qualified Health Claims Requirements for a Health Claim Health claims cannot be made about the diagnosis, cure, mitigation or treatment of diseases (this is a drug claim) They must be complete, truthful and not misleading.
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and































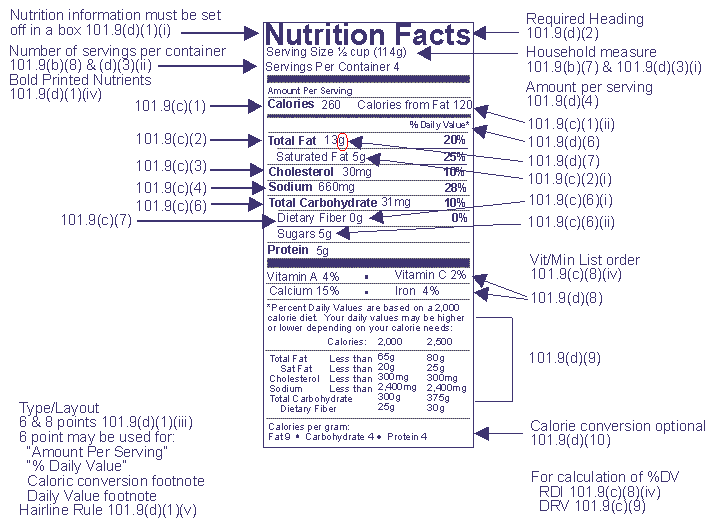
Post a Comment for "44 fda approved health claims on food labels"